Background:
Financial toxicity (FT) is defined as financial distress as a result of disease or treatment decisions. FT might be considered analogous to physical toxicity. Patients with high risk hematologic malignancies represent an especially vulnerable group for cost related issues due to heavy healthcare utilization, high costs associated with their treatment, and the potentially deadly consequences of treatment noncompliance. We hypothesized that comprehensive intervention on the financial aspects of care for these patients would lead to decreased mortality.
Methods:
All patients seen in Leukemia Clinic at the Levine Cancer Institute, a tertiary hospital-based specialty practice, were surveyed prior to their visit over a six-month period. All patients were aged ≥18 years and diagnosed with a high-risk hematologic malignancy (intermediate/high risk AML, high risk ALL, high or very high risk MDS, blast phase CML, or mixed phenotypic acute leukemia). The survey consisted of the PROMIS Global-10 measure and two questions from the COST measure. FT was defined as scoring 5 or less (maximum: 10) in agreement with the COST questions: "I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment" and "I am satisfied with my current financial situation." Patients who met these criteria were entered into the interventional cohort and scheduled for comprehensive intervention based on availability of study staff. Scheduling was done anonymously and without prioritization of perceived need. The intervention consisted of a nurse navigator visit where patients completed a standardized worksheet to identify gaps in care and opportunities for grant funding/other assistance, a clinical pharmacist visit for medication copay review and discussion of assistance programs, and the services of a community pro-bono financial planner for assistance with budgeting, asset management, and general financial advice. Demographic/clinical data were abstracted from the medical record and patients were tracked longitudinally for clinical outcomes. Categorical factors were summarized with proportions and compared between groups with Fisher's exact tests, while continuous factors were summarized with medians and compared between groups with median two-sample tests. Overall survival (OS) was evaluated with Kaplan Meier methods and compared between groups with a log rank test. Cox proportional hazards models were utilized in model selection procedures for OS. Individual prognostics were identified with univariable models, then backward elimination and forward selection (entry/elimination criteria p = 0.10) were carried out to identify a final base model. This was utilized to estimate an adjusted HR for the intervention variable.
Results:
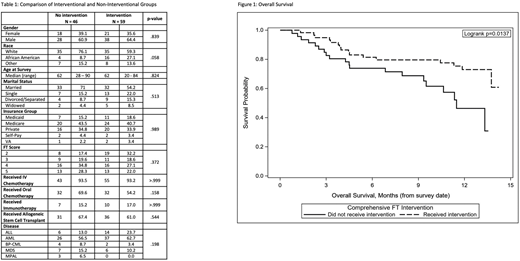
A total of 105 patients met criteria and were placed in the interventional cohort with the intention to receive the full intervention. Of these patients, 59 (56.2%) were able to be scheduled and receive the full intervention, while the remainder received standard care only. There were no significant differences between the groups when compared by gender, race, age, marital status, insurance type, FT screening score, therapy received, or disease type (Table 1). OS was significantly different between the two groups, with a mortality rate during the study period of only 27% for the patients that received the full FT intervention as opposed to 43% for the patients who received standard care. Adjusted OS at 6 months for the interventional cohort was 81.4% (95% CI 68.9%-89.2%) versus 73.9% (95% CI 58.7%-84.3%) for the non-intervention group; OS rates at 12 months were 73.0% (59.0%-82.9%) and 46.4% (28.9%-63.8%), respectively (Figure 1).
On univariate analysis, intervention was significantly associated with survival (HR: 0.44, 95% CI: 0.22 - 0.86, p = 0.017). After adjusting for insurance, race, and age at survey, risk of death in those receiving the intervention was 0.47 times the risk of death in those without the intervention (95% CI: 0.23 - 0.98, p = 0.043).
Conclusions:
High risk hematologic malignancy patients are at high risk for increased complications due to financial concerns. Intervening on FT in a comprehensive way including navigators, pharmacists, and financial counselors is effective and leads to decreased mortality.
Knight:Foundation for Financial Planning: Research Funding. Ai:Incyte: Speakers Bureau; Celgene: Speakers Bureau. Chojecki:Incyte: Research Funding; Novartis: Other: Investigator Meeting Attendance. Copelan:Amgen: Membership on an entity's Board of Directors or advisory committees. Grunwald:Pfizer: Consultancy; Agios: Consultancy; Abbvie: Consultancy; Agios: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Cardinal Health: Consultancy; Incyte: Consultancy, Research Funding; Trovagene: Consultancy; Daiichi Sankyo: Consultancy; Astellas: Consultancy; Daiichi Sankyo: Consultancy; Trovagene: Consultancy; Abbvie: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Merck: Consultancy; Abbvie: Consultancy; Celgene: Consultancy; Janssen: Research Funding; Trovagene: Consultancy; Premier: Consultancy; Astellas: Consultancy; Astellas: Consultancy; Genentech/Roche: Research Funding; Premier: Consultancy; Premier: Consultancy; Amgen: Consultancy; Cardinal Health: Consultancy; Incyte: Consultancy, Research Funding; Celgene: Consultancy; Genentech/Roche: Research Funding; Genentech/Roche: Research Funding; Forma Therapeutics: Research Funding; Incyte: Consultancy, Research Funding; Merck: Research Funding; Janssen: Research Funding; Forma Therapeutics: Research Funding; Forma Therapeutics: Research Funding; Pfizer: Consultancy; Pfizer: Consultancy; Merck: Consultancy; Amgen: Consultancy; Merck: Consultancy; Cardinal Health: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal